Section 1: Biologic Drugs: Then and Now
 Understanding the history of the development of biologic drugs can help to frame the tremendous change the pharmaceutical industry has undergone in the past 40 years. In this time these drugs have evolved from experimental treatments for deadly diseases and conditions, into highly engineered therapeutics which are regularly prescribed treatments.
Understanding the history of the development of biologic drugs can help to frame the tremendous change the pharmaceutical industry has undergone in the past 40 years. In this time these drugs have evolved from experimental treatments for deadly diseases and conditions, into highly engineered therapeutics which are regularly prescribed treatments.
Definition
Biologic drugs (we will refer to them as “biologics”) are therapeutic peptides or proteins, which are produced through the metabolic activity of living plant, animal, bacterial, or human cells. Biologics are produced in many shapes and sizes, though essentially all biologics are larger, and much more structurally complex, than the class of drugs known as small molecule drugs, like Aspirin® (Bayer) or Lipitor® (Pfizer).
Biologics are not produced by chemists in a lab: scientists typically insert the genetic code for a desired biologic into live cells, which are then “programmed” to produce the biologic drug. Note that there are also naturally occurring biologics, such as hormones, that can be naturally isolated from specific cells.
The resulting biologic is:
- Complex
- Sensitive to production conditions
A Brief History
 The introduction of recombinant DNA technology allowed scientists to isolate a gene of interest, engineer it to suit their purposes, and insert the gene into a cell line which could be manipulated to produce the biologic in large quantities.
The introduction of recombinant DNA technology allowed scientists to isolate a gene of interest, engineer it to suit their purposes, and insert the gene into a cell line which could be manipulated to produce the biologic in large quantities.
The first biologics produced were relatively simple (i.e. small hormones, with relatively simple structures), but with current technology we can produce large antibodies which are heavily engineered to create the desired structure and function.
A Biologic History
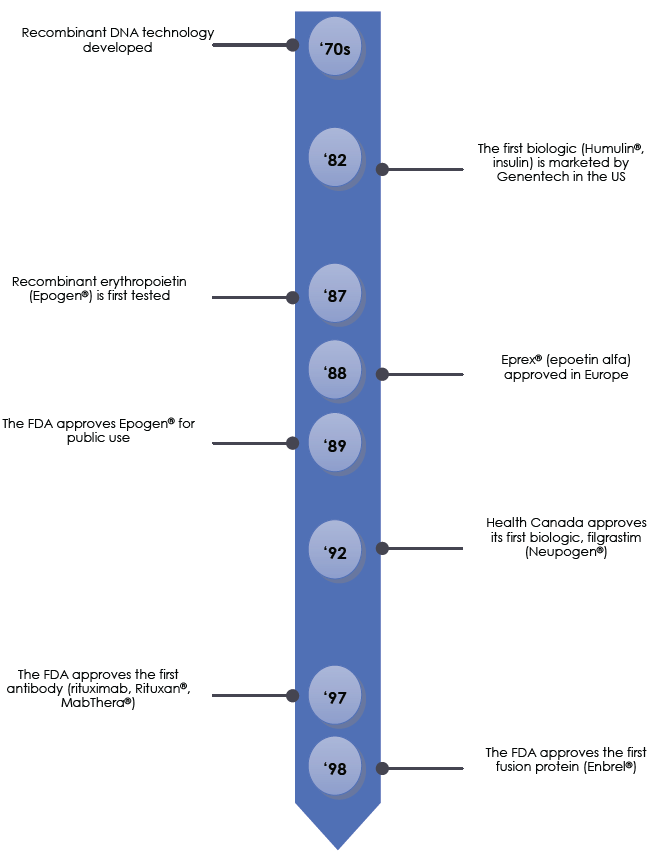
 The timeline on page 7 highlights the major advances in the production of biologics, along with some of the relevant regulatory milestones. The field of biologic drugs has evolved rapidly over the past 40 years, with researchers and regulators continually adapting to advances in scientific techniques.
The timeline on page 7 highlights the major advances in the production of biologics, along with some of the relevant regulatory milestones. The field of biologic drugs has evolved rapidly over the past 40 years, with researchers and regulators continually adapting to advances in scientific techniques.
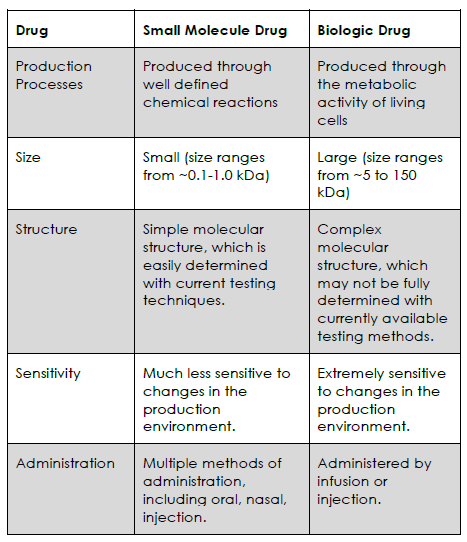
Throughout this Section, we examine the differences between biologics and small molecule drugs in greater detail. These important topics will provide the foundation for understanding the difference between biologics (Reference/innovator biologics and biosimilars), small molecule drugs and its generics.
A Comparison to Small Molecules
In order to understand why biologic drugs are so complex, you will first need to understand what differentiates a biologic from a small molecule drug. The key differences are found in:
- Production
- Administration
- Purity
- Size
Let’s take a look at each of these in greater detail now.
Production
A critical distinction between biologics and small molecule drugs is how they are made: small molecule drugs are synthesized through highly controlled chemical reactions (a process that converts one set of molecules into a different set of molecules, as shown below). On the other hand biologics are the product of a living system of cells, which are grown in a specific growth medium, and under specific conditions (temperature, pH, pressure, etc.).
Synthesizing a Small Molecule Drug
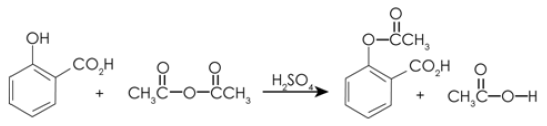
Small molecule drugs are relatively straightforward to synthesize de novo (from scratch), given their small size. However, creating a therapeutic protein through de novo synthesis is not presently an economically feasible way of producing a biologic. Harnessing the metabolic activity of cells provides a more cost-effective method of producing biologic drugs.
 Administration
Administration
Once produced, small molecule drugs can be processed into ingestible tablets or capsules. When consumed, the drug is released in the gastrointestinal tract, where it is absorbed into the bloodstream and circulated throughout the body. Given their small size, small molecule drugs can typically cross cell membranes, and target the molecular “machinery” inside the cell, without the help of carrier proteins.
In contrast to small molecule drugs, biologics cannot be taken by ingestion, since they would simply be broken down with the rest of the protein in the patient’s diet. Instead, biologics are administered by injection or infusion, and must be carefully designed to interact correctly with the body’s endogenous proteins and cofactors.
Purity
Another important distinction between biologics and small molecule drugs is the level of purity that can be expected in the final product. Scientists are able to easily evaluate the purity of small molecule drugs, and compare the molecular features of any contaminants. Unfortunately, this level of analysis is not possible for many biologics, which are simply too large and complex to be perfectly analyzed with current scientific techniques.
Comparison of Size: Two Different Worlds
Another important difference between biologics and small molecule drugs is their size: small molecule drugs are a tiny fraction of even the smallest biologics, as shown below:
Biologics Compared to a Small Molecule Drug
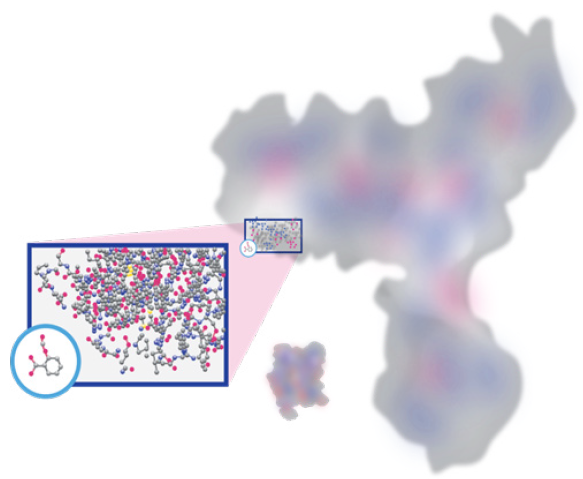
Adapted from [5]
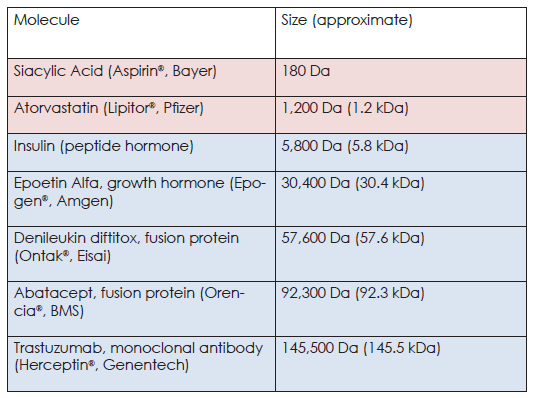
To put the image on page 10 into perspective, the aspirin molecule (circled in blue) has a mass of ~180 Da (and only 21 atoms), while the antibody molecule has a mass of ~150,000 Da. Even the small biologic in the picture (insulin) is ~30 times larger than aspirin!
To further highlight the differences in size, we have included some common examples of small molecules (pink) and biologics (blue) in the table below.
A Comparison of These Molecules

 With Size Comes Complexity
With Size Comes Complexity
The large difference in size results in the biologic having a much more complex molecular structure and functionality (covered later in this Module), as compared to the small molecule.
For larger biologics, the increase in size exponentially increases the number of sites where changes can occur, affecting the structure and function of the drug. These changes can occur at various levels, including:
- Changes to the primary sequence of amino acids through mutation of the genetic code, or errors in how the amino acids are joined together.
- The addition of sugar molecules to the primary sequence (through a process called glycosylation).
- The addition of various other chemical groups to the primary sequence.
- Cellular processing that removes part of the protein.
- The formation of new structural bonds between different sections of the protein.
Structural Heterogeneity
Each of these structural changes is performed by a complex set of molecular “machinery” within the cell, which cannot be perfectly controlled in the large cell cultures (often 1000s of litres) used to produce biologics.
Minor structural variants are to be expected, meaning that the final product is a heterogeneous mixture of similar proteins, with subtle molecular differences. In contrast, small molecule drugs with even minute structural differences would likely fail to obtain regulatory approval.6
The largest biologics (e.g. antibodies) are typically the most complicated to produce, since their sheer size requires large amounts of testing to ensure they are safe for use in humans. These products are most likely to contain structural heterogeneity, and are extensively monitored to ensure that adverse events are not linked to changes in the molecular structure of the biologic.
A Primary Sequence

Adapted from [7]
The Folded Product
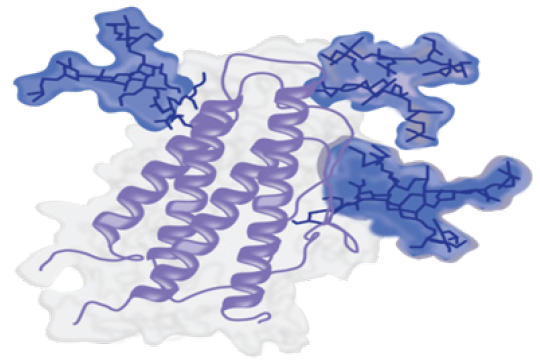
As you can see in the images above, the structure of a biologic starts as a relatively simple sequence of amino acids. However, the final folded product contains changes and additions (e.g., glycosylation highlighted in blue), and has formed an intricate three-dimensional structure.
The structure of a small molecule drug is well defined, and easily controlled, which makes them relatively simple to produce. In contrast, biologic drugs are larger, with each having its own unique three dimensional structure.
 Biologics: Inherently Complex
Biologics: Inherently Complex
Consider a friend that is a connoisseur of fine wine (or scotch, food, art, etc.). They are so intimately familiar with their field of interest that they can detect the most minor variations in bouquet, taste, and style. Your body’s immune system is even more exquisitely sensitive to the molecular structure of proteins and small molecules!
The human immune system is exquisitely “calibrated” to detect foreign molecules (by noticing differences in molecular structure), which means that even small changes in the molecular structure of a biologic can have consequences when the drug is given to patients. Biologics are expensive to develop, since the amino acid sequence of the biologic is just the “backbone” of the drug, with additional molecules often being added during production. These additional molecules may cause immune reactions, often referred to as immunogenicity. The concept of immunogenicity will be covered later in this course8.
These additional molecules (e.g. post-translational modifications) are added inside living cells, meaning that manufacturers rarely have absolute control over the final product (though proper quality checks can assure a fair degree of similarity). Cells can modify the proteins they produce in a variety of ways, from minor atomic changes (10-100 Da) to substitution of large sugar groups (>1 kDa).
The research and development of a biologic drug is focused on ensuring its safety and efficacy, this is done by designing the molecular features that are compatible with the human immune system. Later in this program we examine how biologics are “engineered” to ensure their safety and efficacy. For now, just remember that biologics are inherently more complicated than small molecule drugs.
As you can see in the table below, small molecule drugs are much simpler, and easier to produce, than biologic drugs.
The Complex Nature of Biologics
Biologics on the Market Today
With their structural complexity, biologics are produced for use in a variety of diseases and conditions, and can range from simple protein fragments to large monoclonal antibodies.
Each of these biologics will contain at least one function domain (often two or more), and will most likely be subjected to complex structural modifications,5,10. Once these products have adequately been tested to ensure they are safe for use in patients, they will receive regulatory approval from Health Canada for marketing in Canada. This is referred to as a Notice of Compliance, or NOC.
Examples of biologics that have received NOC from Health Canada include:
Enzymes:
- Pulmozyme® (dornase alfa, Genentech) to treat Cystic Fibrosis
- Elaprase® (idursulfase, Shire) to treat the enzyme deficiency that causes Hunter Syndrome
Immunomodulators:
- Betaseron® (interferon beta, Bayer) to treat inflammation in the brain for patients suffering from Multiple Sclerosis
- Kineret® (anakinra, Amgen) to treat inflammation in patients with Rheumatoid Arthritis
Growth Factors:
- Leukine® (sargramostim, Wyeth) to treat patients who are suffering from acute myeloid leukemia
- Kepivance® (palifermin, Amgen) to prevent mucositis in patients with blood cancers who are receiving chemotherapy
Hormones:
- Nutropin® (somatropin, Genentech) to treat patients with growth hormone deficiency
- Recormon® (epoetin alfa, Roche) to treat anemia caused by chronic kidney disease
Vaccines:
- Adacel® (generic? Sanofi Pasteur) to protect against diphtheria, tetanus, and pertussis infections
- Fluviral® (generic? GlaxoSmithKline) to protect patients against the seasonal influenza virus
Monoclonal Antibodies:
- Rituxan® (rituximab, Roche) to treat autoimmune disorders like leukemia, lymphoma, and rheumatoid arthritis
- Humira® (adalimumab, AbbVie) to reduce inflammation in autoimmune disorders like rheumatoid arthritis and Crohn’s disease
Monoclonal Antibodies (mAbs) are typically considered a unique class of biologics, based on their large size and complex functionality. We will examine this class of biologics in more detail later in this program.
Now that we’ve reviewed how different small molecule drugs and biologic drugs are, let’s move on and look at the various steps involved in producing a biologic.
 In Summary
In Summary
Biologic drugs, or biologics, are therapeutic peptides or proteins which are produced through the metabolic activity of complex systems of living cells (bacterial, human, animal, etc.).
Biologics are structurally complex, difficult to fully characterize, and often quite sensitive to production conditions.
In contrast, small molecule drugs are synthesized through controlled chemical reactions, and are relatively simple to purify and characterize.
The major difference between biologics and small molecule drugs is their size: biologics are typically ~30-150kDa, while small molecule drugs are a small fraction of that.
The larger size of biologics gives them a more complicated molecular structure and function(s).
The amino acid sequence of a biologic is just the “backbone”, with further structural modifications often being added in the cell to achieve full functionality.
 Takeaway Gems
Takeaway Gems
All drugs are unique. Size, complexity, and molecular mechanisms affected all play a role in determining how well patients respond to specific drugs.
Small molecule drugs are relatively simple, while biologic drugs are more complicated to manipulate and produce. The complexity of a biologic drug’s structure and function require an intricate production process, which is tailored to ensure the drug’s safety and effectiveness.
We can’t say that small molecule drugs are better or worse than biologic drugs. They’re just… different.
