MODULE 1: STRATEGIC DECISION POINT 1: DEVELOP THE ORGANIZATION
Module Overview
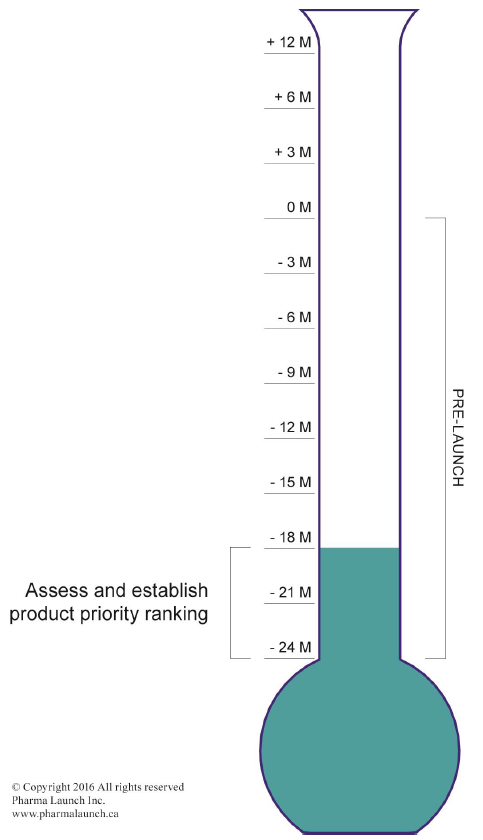
This module explores how a company determines the potential market opportunity of a drug and the subsequent resource allocation and initial planning that is required at the earliest stage of the launch process. It outlines the full range of tasks that must be performed at twenty-four months pre-launch, with a focus on developing the organization.
Each task includes:
- Timeline in the process
- Team(s) responsible
- Desired output
- Planning recommendations
Module Objectives
Upon completion of this module, you will be able to discuss:
-
- The critical role of a commercial assessment in the company’s decision to launch a product
- How brand ranking is essential to the allocation of resources and the achievement of launch excellence
- The importance of thorough preparation to ensure the product has the potential to achieve peak sales
- The various tasks and action items required at the earliest stage of the launch process
STRATEGIC DECISION POINT 1: DEVELOP THE ORGANIZATION
The key questions to answer at Strategic Decision Point 1 are:
- How large is this opportunity?
- How many resources do we invest?
-
- Rank new product priority.
- Build a launch team.
- Communicate the importance of the product within the organization.
- Complete the regulatory review of the file.
- Assess favourable pricing alternatives.
Tasks Initiated at Twenty-Four Months Pre-Launch
Every product launch brand has the potential to achieve world-class status. However, without careful preparation, any launch brand can end in failure. The mantra of Pfizer, a Top-10 pharmaceutical company, is “Blockbusters are made,” which is entirely true, as is the reverse.
The launch team must rely on the product they are commercializing as it impacts customers’ understanding of it, the payor’s willingness to pay for it, and the patient’s willingness to consume it. Without thoughtful preparation in the twenty-four months prior to launch, gaps in key areas will prevent the product from achieving peak sales.
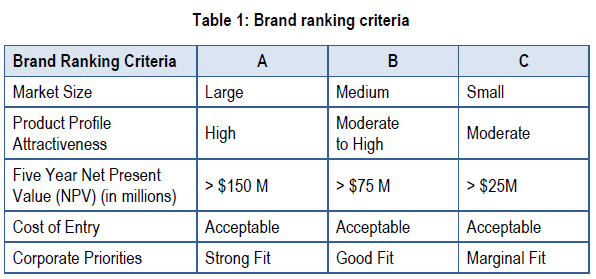
The first decision point in the framework involves developing the company, when it decides how to resource the branding appropriate to the size of the opportunity. At this decision point, the team completes a commercial assessment of the opportunity and identifies the product’s key issues. The team then provides senior management with a ranking assessment, such as an “A,” “B” or “C” brand priority. An “A” brand represents a large opportunity, whereas a “C” brand would represent a lesser opportunity but still be financially viable.
The team involved in this stage consists of: GM, Regulatory, Finance, Marketing, Legal, Market Access and Market Research.
Strategic Decision Point 1 requires a detailed commercial assessment to establish the company’s brand priority ranking, crucial to prioritize it amongst other projects. An “A” brand, for example, would be given top priority for internal resources, whereas a “C” brand might receive a smaller budget and more external resources. Brands may change rankings over time as new information become available. A brand’s ranking should not impact the quality of the preparation for the launch. Decision criteria are provided below for all rankings.
The purpose of ranking brands is to ensure that resources are assigned appropriately according to the magnitude of the business opportunity. A company may have to stretch its personnel and finances if it has too many brands competing for its limited resources. Failing to establish priorities may prevent a company from reaching launch excellence for any of its brands. Lower brand rankings may still be vitally important to the company, but they require different skills and tools to achieve success, such as the use of contracted or partner resources.
By ranking product priority for each product’s development, an optimal structure is established. As a general rule, structure follows strategy.
Author’s Launch Insights
While launching three brands simultaneously in an organization, determining launch priorities became a challenge. When we assessed the products from a strategic perspective and their contribution to the organization’s specialty focus versus simply the financial contribution on an annual basis, priorities became clearer. Two brands were aligned with our corporate specialty focus and a third brand was offering primary care a superior treatment – a one-off product! As a result, it did not deserve the same resources as products aligned with the corporation’s specialty area. Ultimately, despite a lower priority, the primary care product still became the number one treatment within its therapeutic area.
The following table lists the tasks that need to be performed in the earliest launch stage, with a primary focus on developing the company. Each task will be covered in detail in this section. The table lists the groups that are chiefly responsible for implementing each task. The last column provides the timeline outlining when each task should be initiated.
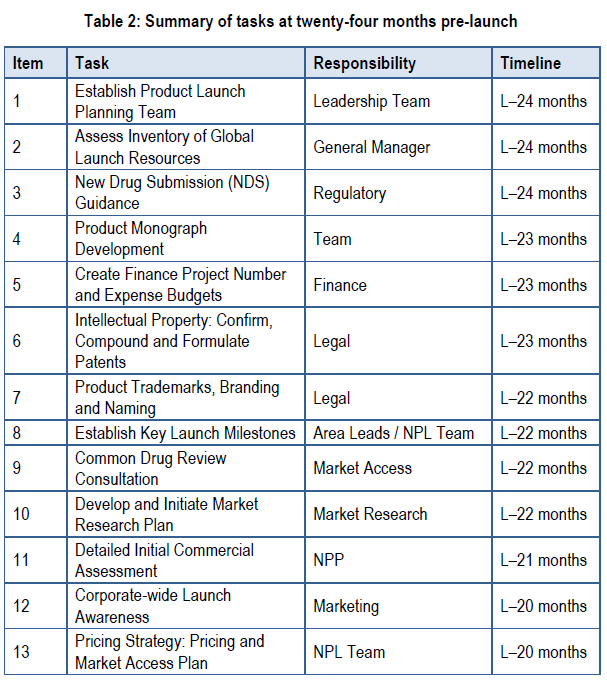
The following section provides detailed information on the recommended tasks required to develop the company to achieve launch excellence.
Task 1:
Establish Product Launch Planning Team
Responsibility for Decision: Corporate Leadership Team
Output: Establish the core cross-functional launch team.
Planning Recommendations:
The first step in the launch process is the kick-off meeting during which senior management introduces team members and their primary responsibilities. At this meeting, the newly created, cross-functional New Product Launch (NPL) team will assess the tasks to be initiated and enlist the appropriate stakeholders for each task. An important step in launch team development is to communicate early when new members will join the team.
The six-stage strategic decision and check-in points provide an excellent overview to inform team members at which point(s) their expertise will be required, which will help to motivate them as they are well informed.
Successful product development demands a cross-functional approach beginning in the earliest, pre-clinical stages prior to the product’s transfer to new product marketing. Early-stage commercialization staff often participates in teams with business development, research and development (R&D), medical affairs, regulatory, legal, clinical, supply and others. A list of these teams and their functions will be covered below and can be found in the Appendix. Cross-functional product development processes ensure that decisions are well thought out and that checks and balances are in place as perspectives from across the company are included.
Within the NPL team, a product manager is responsible for the short-and long-term profitable progress of the brand in the marketplace through effective planning, execution, control and training. Regulatory is responsible for all activities involving the submission and receipt of approval from Therapeutic Product Directorate (TPD). Finance is responsible for all financial statements and budgetary controls. Supply and quality assurance (QA) are responsible for providing the final product for sale that meets good manufacturing practices (GMP). Market access is responsible for gaining approval on formularies to allow the brand to reach its fullest potential. Finally, legal is responsible for ensuring that contract provisions, trademarks and patents are in place to maximize the product’s life cycle.
Initially, keep the cross-functional launch team small. Include only the individuals required at each stage of development, using the checklists provided at the beginning of each stage. Multiple ad hoc teams can be formed beyond the core team for specific short-term and long-term projects. Meetings should be scheduled monthly or bi-weekly in the beginning to facilitate the flow of information and expedite product planning. Each area of the launch team should be responsible for writing its section of the plan.
To give a product the best chance at long-term success, priority must be given to developing the launch team. When considering a product’s launch and life cycle, solicit input from all functional areas, whether your organization establishes a cross-functional NPL team or passes responsibilities from function to function. Functional ownership may actually speed decisions. Cross-functional launch team collaboration ensures access to the knowledge and experience of multiple divisions and departments.
Task 2:
Assess Inventory of Global Launch Resources
Responsibility for Decision: General Manager and Business Development Team
Output: Key files and documents are made available to new product launch (NPL) team.
Planning Recommendations:
Once the transfer of the new product from the business development team is completed, the commercial team (including the NPL team) has the “green light” to begin planning. Whether the new product is an acquired product or developed within the company, the business development team has invested considerable resources into its assessments, thus a thorough review of all business development files is warranted to quickly augment the commercial team’s familiarity with the product. The NPL team needs access to the due diligence process files, key assumptions, forecasts, commercial plans, pivotal clinical trials, partner agreement (which includes term sheets), the international launch sequence, international pricing guidelines, conditions, Therapeutic Product Directorate (TPD) file status, product supply lead times, branding and trademark information, Intellectual Property (IP), market research and Life Cycle Management (LCM) plans.
Business development assessments are essential to understanding the opportunity presented to the NPL team and serve as a fast-track foundation for effective planning. They provide an invaluable perspective on the product’s potential in the market.
Author’s Launch Insights
The transfer of all files from the business development team is important to understand the product’s development, anticipated market opportunities and licensing arrangements. It is also important for the business development team to assess the priorities of all development brands. In one organization, the business development leader believed that sharing the product files would not allow the product development team to develop its own ideas for the brand, but contributing to product development ought to be an organization-wide initiative. Gaining perspective from all team members will broaden a product’s strategic options and make a product more successful.
Task 3:
New Drug Submission (NDS) Guidance
Responsibility for Decision: Regulatory
Output: Plan and execute pre-NDS meeting and follow-up with Therapeutic Product Directorate (TPD) for clinical program and submission guidance.
Planning Recommendations:
To give a new product its best chance at success, a focused effort on rapid approval from TPD is paramount. The NDS submission is the most important part of the launch. Without a timely submission, review and approval, the product does not launch.
The groundwork for the NDS must be meticulous in every way; all information and documentation must be assembled as required by the TPD with extreme diligence. Key components include: efficacy and safety, chemistry and manufacturing, non-clinical and clinical data. The pre-clinical stage of research initiates prior to clinical trials, to demonstrate drug safety. The clinical trials focus on testing the drug in humans.
There are few hard and fast rules for NDS development, but some pertinent guidelines may help facilitate preparation. TPD Guidelines can be obtained through the Health Canada Website (Keywords: Health Canada, NDS) or directly from TPD, Bureau of Pharmaceutical Assessment, Finance Building, Tunney’s Pasture, Ottawa, Ontario, K1A 1B6.
Before NDS preparation begins, determine if FDA or ICH guidelines may be relevant to the NDS. In some cases, the U.S. Food and Drug Administration (FDA) has more regulatory guidelines than does TPD. These should be reviewed for relevance. The International Conference on Harmonization (ICH) is working to standardize regulatory requirements in Europe, Japan and the U.S. and TPD has adopted these guidelines. Prior to moving too far ahead in the NDS’s development, a pre-NDS meeting with the reviewers at TPD is common.
Prepare a pre-meeting package containing a summary of all clinical and technical data to be used in the preparation of the NDS. Members of the regulatory and medical teams present the data that will be submitted to support the NDS. Outstanding issues or concerns raised by the reviewing unit can be clarified and negotiated at the meeting.
Task 4:
Product Monograph Development
Responsibility for Decision: Regulatory, NPP, Medical and Marketing
Output: A product monograph (PM) is aligned with commercialization objectives.
Planning Recommendations:
Development of a well-crafted product monograph is critical to a product’s success since the wording in the product monograph influences the degree of success or failure for a new product. Make all attempts to include relevant information including key disease and brand messages in the product monograph because it is essential for future promotional purposes.
The responsibility for NDS preparation resides with the regulatory and medical teams with input from New Product Planning (NPP) and marketing teams in regards to the information that is critical to the new product’s ability to compete.
Prior to preparing the product monograph, assess competitor products for class labelling guidelines that may apply directly to the new product. Review competitive monographs for style and content, especially those that provide the specific products’ competitive advantages. All product monograph statements and claims must be supported in the submission by literature and data. Failure to provide sufficient support may delay approvals. Another valuable suggestion is to gain Key Opinion Leader (KOL) perspectives on the key drivers of prescribing within the product’s treatment area, as well as to include product differentiation in order to support future promotional efforts. A recommendation is to hire a consultant to provide a competitive product monograph review. The person developing the product monograph review needs a strategic skill set to recognize competitive promotional opportunities and the long term promotional environment in Canada. The product monograph is the most important competitive document in the product’s life cycle.
Author’s Launch Insights
A well-referenced product monograph is vital to a product’s promotional success. Be sure to look five to ten years down the road to determine where your product’s promotional opportunities lie. To achieve this objective, use an outside marketing consultant with both regulatory and commercialization expertise for an in-depth competitive product monograph assessment and review.
Task 5:
Create Project Number & Expense Budgets
Responsibility for Decision: Finance
Output: Set up a project number for the product, as well as the preliminary expense budget and budget codes.
Planning Recommendations:
It is financially prudent to establish a project number to efficiently track expenses. When the product is transferred from business development to the launch team, it is important to calculate the preliminary expense estimates for the next three to six months. A project number, set up with your financial partner and all launch team members, will track the product expenses. Although actual product codes will eventually be created, all expenses in the short-term need to be allocated to a project number for streamlined organization. It is recommended to involve your financial team early as key partners in the product’s development.
Task 6:
Intellectual Property: Confirm Compound, Formulation Patents & Distribution Rights
Responsibility for Decision: Legal and Cross-functional Team
Output: Patent review for intellectual property (IP).
Planning Recommendations:
File applications for patents early to protect market exclusivity. As part of the business opportunity review, include a review of the patent standing for the new product, whether it is an in-house R&D or a licensing opportunity.
Some key points to consider:
- Review Orange Book to verify any patent registration numbers, and, where required, report on any patent information received from licensing partners.
- All dosages and formats used in any clinical trials must be patent-protected.
- Confirm the patent status with your company patent agents.
- Patents related to a new medicine should also be registered with Health Canada. This step is not mandatory but should be considered as a deterrent to premature generic applications. It is recommended to register all issued patents with Health Canada when the NDS is submitted for review.
- Be certain to verify if there are any competitive patents that should concern your company.
Both compounds and formulations must carry patents. At this stage, the active pharmaceutical ingredient (API) or compound for the new product should already have a registered patent. Health Canada maintains a patent register, linked to Notices of Compliance, forcing any generic manufacturer to notify the patent holder by way of Notice of Allegation (NOA), prior to filing an abbreviated new drug submission. The innovator can then institute court proceedings to prevent the issuance of an NOC to the generic manufacturer while the patent is in place. Strict conditions apply to the entry of a patent on Health Canada’s register.
When patents are registered, the legal team should verify that:
- Only patents for a medicine or use of a medicine can be registered (chemical process patents cannot be registered with Health Canada).
- All inventions relating to the product are the subject of patent applications filed before the NDS is made.
- All issued patents must be submitted for registration at the same time as the NDS.
- All patents relating to the product must be re-submitted for registrations whenever a supplemental NDS is made.
- Any new patents issued after submitting the NDS must be submitted for registration within thirty days of their issuance.
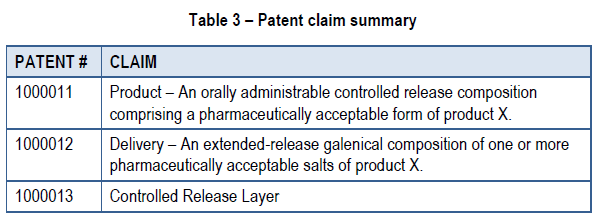
The IP status defines the lifecycle timeframe of the brand. Several intellectual patent claims exist, such as:
Claim for the medicinal ingredient – is made in the patent, whether chemical or biological in nature, when it has been prepared or produced by the methods or processes of manufacture that are described and claimed in the patent or by their obvious chemical equivalents. It also includes a claim for different polymorphs of the medicinal ingredient, but does not include different chemical forms of the medicinal ingredient.
Claim for the formulation – a claim for a substance that is a mixture of medicinal and non-medicinal ingredients in a drug that is administered to patients in a particular dosage form.
Claim for the dosage form – a claim for a delivery system for the administration of a medicinal ingredient in a drug or a formulation of a drug that includes within its scope that medicinal ingredient or formulation.
Claim for the use of the medicinal ingredient – a claim for the use of the ingredient for the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state or for its symptoms.
An example of a patent claim summary is provided in the table below for Product X.
Distribution rights for a licensed product define the geographical territory for sales and marketing of a product. Understanding constraints is important, such as a stipulation on number of calls annually.
Task 7:
Product Trademarks, Branding & Naming
Responsibility for Decision: Legal, Business Development, Regulatory and NPP
Output: Brand name established, trademarks identified and registered.
Planning recommendations:
Be sure to use the appropriate trademarks on all promotional materials. All trademarks should be clearly identified with the TM symbol. Once registered, the symbol can remain TM or be changed to ®. Trademarks are used to identify products and to distinguish them from competitors’ products.
There are three general types of trademarks, and all three can apply to your new product:
- Word Mark – usually a brand name. (i.e. Mercedes-Benz, SUNOCO)
- Design Mark – or logo. (i.e. Bayer cross, the Mercedes-Benz three-point star)
- Trade Dress – the shape and colour of a tablet or capsule; the shape, configuration and colour of a dispensing device. (i.e. Coca-Cola bottle)
Customers will identify a product by factors such as its name, colour, shape and size, and these trademarks are perceived as an indication of quality. Marketing management should be involved in the selection of trademarks for the product, and the trademarks should be registered with the Canadian Intellectual Property Office.
Registration of a trademark gives the manufacturer an exclusive right to use the trademark across Canada. The application process often takes a year or more, thus it is important to file it at this stage in the process.
The Canadian Intellectual Property office has an excellent guide to help you understand trademark selection and use. It can be accessed on their website (Keywords: Canadian Intellectual Property – Trademarks).
Author’s Launch Insights
Your communications team must follow your trademark guidelines or you risk weakening your trademark. In one organization I served, prior marketers failed to use trademark notations, which led to competitors using a similar trademark claim. As a result, the strength of our claim was weakened due to a lack of diligent TM symbol use, though we had filed for a registered trademark. Maintaining our trademark would have afforded a competitive difference in the market place, yet we lost this due to carelessness in our advertising and promotional materials. Always reference your trademark, especially prior to receiving your final registered trademark.
Task 8:
Establish Key Launch Milestones
Responsibility for Decision: Functional Area Leaders and Launch Team
Output: Specific, achievable and measurable milestones for launch success.
Planning Recommendations:
Establish challenging goals at the senior level and link results to individual and team performance reviews. The commitment to achieving key milestones for the brand is crucial, as they are recommended by the cross-functional launch team with the functional leads’ and general manager’s final approval. Because many of these milestones are stretch goals, they will need support and alignment from the senior management team.
Examples of launch milestones are:
- Regulatory target dates: prepare NDS file for submission, hold pre-submission meeting with TPD and achieve NOC within X days of receipt of mandate.
- Developing the market: X% of KOLs trained with speaker program prior to NOC.
- Medical Science Liaison team: trained and deployed within X months pre-NOC.
- Phase IV clinical trial program launched at NOC.
- Sales force allocated by X (date) pre-NOC and trained by NOC.
- Trade product available to ship to pharmacies X days after NOC.
- Sample product available to ship to sales representatives within X days of NOC.
- Gain market access in targeted provinces: ON, AB, BC and PQ within X days of launch.
Author’s Launch Insights
To prepare for the launch of a gastrointestinal drug, the leadership team established aggressive milestones for launch. One milestone was to launch the drug within days of NOC. This goal was surpassed and the company launched the drug one day post-NOC as a result of teamwork focused on a common goal.
Task 9:
Common Drug Review Consultation
Responsibility for Decision: Market Access
Output: Gain opinion from Common Drug Review (CDR) regarding data gaps in the economic value message of the brand.
Planning Recommendations:
CDR consultations commence early in the process to identify data gaps in the formulary listing process, and they greatly impact decisions on provincial formulary listings and subsequent market access. Realistically, time will not likely permit new trials within a meaningful timeframe to suit the CDR submission, but new analysis of existing data and re-analysis or re-packaging of possible economic messages will still be possible.
Background
The Common Drug Review at the Canadian Agency for Drugs and Technologies in Health (CADTH) is a pan-Canadian process for conducting objective, rigorous reviews of a drug’s cost-effectiveness and clinical and patient evidence. CDR also provides formulary listing recommendations to Canada’s publicly funded drug plans (except in Quebec).
For more information, please refer to the CADTH website (Keyword: CADTH).
Task 10:
Develop & Initiate Market Research Plan
Responsibility for Decision: Market Research
Output: Obtain a 360-degree view of the market opportunity.
Planning Recommendations:
Complete a comprehensive, competitive review of the market including:
- Secondary prescription and sales market analysis (IMS CompuScript, CDH and Brogan data).
- Prescriptions by specialty group (to determine which specialists, if any, play an active role in the market).
- Current treatment algorithms by indication.
- Current reimbursement landscape, which may include competitive pricing and distribution method, public/private reimbursement split by target indication, competitive reimbursement status and hospital formulary status, if applicable.
- Competitive intelligence on key competitors including patent and promotion information and, if possible, on future competitors.
- Review and selection of best analogues for forecasting purposes.
- Preliminary qualitative opportunity assessment research with prescribers (small sample of relevant specialties) and KOLs.
This complete review will help to:
- Identify trends in customer attitudes and behaviours in the market.
- Document perceived strengths and weaknesses of existing products and therapies.
- Identify existing unmet and emerging needs in the market (patient, physician, pharmacist and payor).
- Obtain customer reaction to preliminary product concept.
It is imperative that market research is conducted at the right time in order to provide the greatest value to the product’s development.
Author’s Launch Insights
I suggest a unique approach to ensure that the commercial team understands the importance of market research planning. Ask your market analytics team to provide an overview of the stages of market research to the launch team based on the product’s needs at various stages of development. In addition to other benefits, this eliminates redundancies in spending.
Task 11:
Detailed Initial Commercial Assessment
Responsibility for Decision: NPP and Launch Team
Output: A thorough commercial opportunity assessment and ranking of brand priority.
Planning Recommendations:
Prior to determining a launch “Go” or “No Go” decision while in Strategic Decision Point 1, the NPP and launch team must complete an initial, yet comprehensive, assessment of the brand’s potential, which characterizes the launch in terms of size, critical issues and whether it is a general practice or specialist opportunity.
Author’s Launch Insights
I strongly recommend developing at least your commercial assessment and five-year strategic plan as prose documents, rather than using a haphazard Microsoft PowerPoint. PowerPoint plans do not capture adequate background for future reference. The value in a prose document comes from the detailed background on decision-making that may be useful later. As we all know well, staff turns over quickly in many organizations due to promotions and other opportunities. A detailed document will allow beliefs and assumptions to be recorded. If time and resources are an issue, hire a strategic planning consultant to support this valuable activity.
Task 12:
Corporate-Wide Launch Awareness
Responsibility for Decision: NPL Team and Corporate Communications
Output: Create awareness of the new product and the type of patient treated.
Planning Recommendations:
Once Strategic Decision Point 1 is completed, the team will need to partner with internal Corporate Communications to develop the right internal messaging. To build enthusiasm, a corporate kick-off meeting can be an important initiative. It will create awareness and excitement about the new product opportunity and help to generate support over the next twenty-one months leading up to the launch.
Author’s Launch Insights
For each launch, I offered what I called “product launch town hall meetings” for ease of communication within an organization. These meetings are an opportunity to introduce your product to the entire company and gain support for improved patient care. Holding these meetings on a regular basis builds support and enthusiasm, and tracks product progress too. They are also an opportunity to give your launch team positive exposure in front of the rest of the organization for members’ professional development.
Task 13:
Pricing Strategy: Pricing, Market Access Plan, Confirm Global Price Strategy & Price Corridor
Responsibility for Decision: Market Access
Output: Hold meetings with PMPRB to determine comparators and price tests, and to book meetings with CDR and provincial payors regarding pricing strategy to maximize market access and value.
Planning Recommendations:
Make market access a priority, because it is likely the most important variable in a product’s lifecycle, from its development to the negotiation of an exclusive tendered contract post-genericization.
An important consideration in this area is the global price strategy. We no longer have the freedom we had in the past with a global product launch. Price restrictions, including reference pricing, profit limitations, and price reductions have created a global market with increasing price constraints. Launching in one country may have immediate ramifications in other countries, and pricing decisions can impact other markets despite the best efforts of the company. In this increasingly complex global marketplace, companies must use segmentation analysis, clinical and health outcomes research, parallel trade evaluation, political, economic, social and technological (PEST) analysis, and demand analysis to create a coordinated global pricing strategy that will anticipate regulatory challenges. While extremely difficult to create, an effective, comprehensive global launch strategy more than justifies the costs.
SUMMARY OF STRATEGIC DECISION POINT 1
One of the first steps in launching a product is determining the product’s priority within the company. This is based on the value of potential sales for the product and the investment required for a successful launch. The detailed commercial assessment completed at this stage provides the organization with the required information to make “Go” or “No-Go” decisions in the marketplace. If a product appears to be a “No-Go”, the following questions must be asked: What can be done to make it a “Go”? What plan is required to reach that goal? Considerable resources are invested in the research and development, or acquisition of a licensed product, and the launch team must find a way to successfully market this product.
To complete Strategic Decision Point 1, the following steps must be completed:
- Product has been formally transferred to New Product Planning (NPP).
- New Product Launch (NPL) team leader and team are chosen and are meeting routinely.
- Brand project codes and a preliminary expense budget are set.
- Guidance on the New Drug Submission (NDS) is received and the product monograph is written.
- Canadian patents and trademarks are established.
- Detailed initial commercial assessment is completed.
- A forecast and profit and loss (P&L) assessment has been done.
- Established launch goals and metrics are outlined.
With these milestones reached, the NPL team is at Strategic Decision Point 1. Now, the team, with senior management, will assess:
- Go/Kill: What is required to make the brand a “Go”?
- Brand priority recommendations: A/B/C
- Key brand issues
An outright kill is unlikely at this stage, given an initial “go” decision at 24 months prior to launch. Yet, key issues will remain and should be honestly addressed, such as gaps in the product profile that require additional studies or analysis. Brand prioritization should be assessed by the team to help the organization order its workload.
Parameters for recommendation include:
- Size of the market opportunity.
- Compound attractiveness: including cost-effectiveness (price/reimbursement).
- Risk and probability of success.
- Forecasted costs and return on investment.
- Therapeutic fit within corporate priorities, such as primary care or specialty specifications.
With the elements of Strategic Decision Point 1: Developing the Company addressed and all tasks initiated, the NPL team is prepared to move onto Strategic Decision Point 2: Developing the Product.
Author’s Launch Insights
As you develop communication tools, keep in-mind the time required for French translation. A dosage card may take a few days for translation from English to French, yet a product monograph may require as long as 6 weeks to translate. Build translation timelines into your planning horizon.
